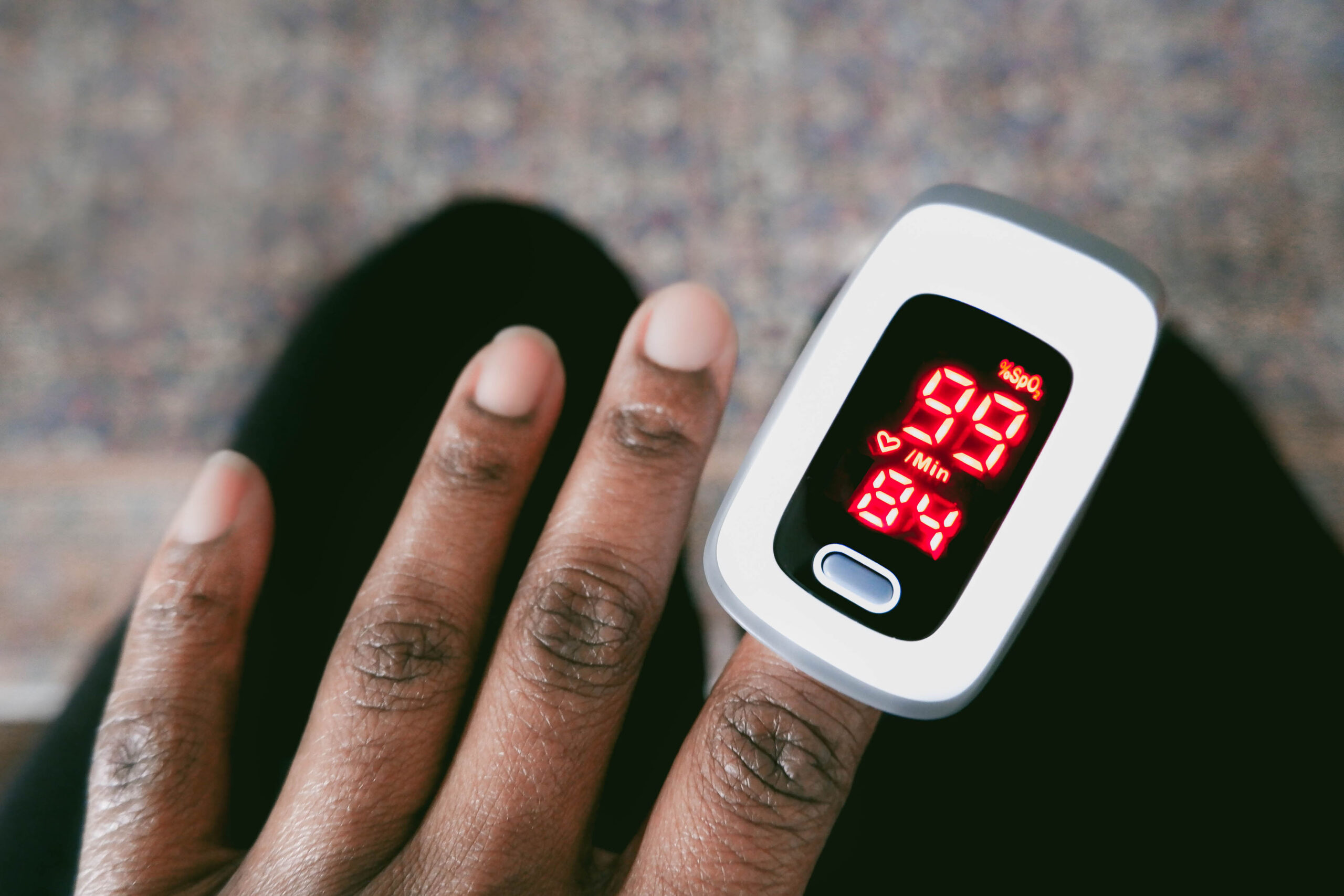
OAKLAND, Calif. — The affected person was in his 60s, an African American man with emphysema. The oximeter positioned on his fingertip registered effectively above the 88% blood oxygen saturation stage that alerts an pressing danger of organ failure and demise.
Yet his physician, Noha Aboelata, believed the affected person was sicker than the machine confirmed. So she despatched him for a lab check, which confirmed her suspicion that he wanted supplemental oxygen at dwelling.
Months later, in December 2020, Aboelata thought again to her affected person as she learn a New England Journal of Medicine article exhibiting that pulse oximeters had been three times as likely to overlook dangerously low blood oxygen ranges in Black sufferers as in white ones. At a time when Black Americans had been dying of covid at excessive charges and hospitals struggled to seek out beds and oxygen for these needing them, the discovering uncovered one of the blatant examples of institutional racism in American well being care.
“I was like, ‘Were there other patients I missed?” stated Aboelata, a household doctor and the CEO of Oakland-based Roots Community Health. As she shared the article with colleagues, “there was so much anger and frustration because we had every reason to believe we could rely on this device, and it was systematically not working in the population that we served.”
State attorneys general and U.S. senators have pressed the FDA to take steps to get rid of pulse oximetry’s racial bias, which has induced delays in remedy and worse well being outcomes, and extra just lately has raised concern concerning the reliability of hospital AI tools that draw on reams of information from the units.
Aboelata’s clinic has sued producers and shops that promote oximeters, demanding they pull the units or add security warnings to the labels. Many of her sufferers depend on dwelling oxygen, which requires correct readings for Medicare to cowl.
But eliminating the units, central to look after coronary heart and lung illnesses, sleep apnea, and different situations, isn’t an choice.
Since the Nineteen Nineties, the handy fingertip clamps have come to face in for a lot of makes use of of arterial blood gasoline readings, that are the gold commonplace for figuring out oxygen ranges however harmful if not finished fastidiously. Makers of oximeters will promote around $3 billion of them this yr as a result of they’re utilized in practically each hospital, clinic, and long-term care facility. During the pandemic, a whole lot of 1000’s of Americans purchased them for dwelling use.
One of them was Walter Wilson, a 70-year-old businessman in San Jose who has had two kidney transplants since 2000. Wilson contracted covid final December however delayed visiting a health care provider as a result of his dwelling pulse oximetry readings had been within the regular vary.
“I’m a dark-complected Black guy. I was very sick. Had the oximeter picked that up I would have gotten to the hospital sooner,” he stated.
Wilson ended up again on dialysis after a number of years of fine well being. Now he’s seeking to be part of a category motion lawsuit towards the machine producers.
“They’ve known for years that people with darker skin get bad readings,” he stated, “but they tested them on healthy white people.”
After years of little motion on the problem, the FDA in 2021 sent a safety warning to medical doctors about oximeters. It has additionally funded analysis to enhance the units and promised to problem new pointers for how you can make them.
But because the FDA polishes draft pointers it had hoped to publish by Oct. 1, clinicians and scientists are uncertain what to anticipate. The company has indicated it is going to advocate that producers check new oximeters on extra folks, together with a big proportion with dark-pigmented pores and skin.
Because of {industry} pushback, nonetheless, the steerage isn’t anticipated to ask machine makers to check oximeters below real-world situations, stated Michael Lipnick, a University of California-San Francisco anesthesiologist and researcher.
Hospitalized individuals are usually dehydrated, with restricted blood stream to their extremities. This situation, often known as low perfusion — primarily, poor circulation — is especially frequent with heart problems, which is extra prevalent in Black sufferers.
Pigmentation and poor perfusion “work together to degrade pulse oximetry performance,” stated Philip Bickler, who directs the Hypoxia Research Lab at UCSF. “During covid, Black patients showed up sicker because of all the barriers those patients face in accessing health care. They’re showing up on death’s door, and their perfusion is lower.”
The FDA steerage isn’t anticipated to require producers to measure how effectively their units carry out in sufferers with poor perfusion. All which means the FDA’s efforts might result in units that work in wholesome dark-skinned adults however do “not fix the problem,” stated Hugh Cassiere, who chairs a panel for the FDA’s Medical Devices Advisory Committee, at its February assembly.
Noha Aboelata, a household doctor and the CEO of Oakland-based Roots Community Health, stated “there was so much anger” amongst her colleagues once they realized that the heart beat oximeters that they had relied on had been “systematically not working” on Black sufferers.(Arthur Allen/KFF Health News)
A History of Inaction
Although some current industry-sponsored research have proven that sure units work throughout pores and skin tones, analysis courting to the Nineteen Eighties has discovered discrepancies in pulse oximetry. In 2005, Bickler and different scientists on the Hypoxia Lab revealed proof that three main units constantly did not detect hypoxemia in darkly pigmented sufferers — particularly those that had been severely oxygen-depleted. Noting that these readings might be essential to directing remedy, the authors referred to as for oximeters to hold warnings.
The FDA’s response was modest. Its regulatory pathway for pulse oximeters clears them on the market so long as they present “substantial equivalence” to units already in the marketplace. In a 2007 draft steerage doc, the FDA prompt that exams of recent oximeters might “include a sufficient number of subjects with dark skin pigmentation, e.g., 30%.” However, the ultimate steerage, issued in 2013, beneficial “at least 2 darkly pigmented subjects or 15% of your subject pool, whichever is larger.” The research had been required to have solely 10 topics. And the company didn’t outline “dark-pigmented.”
Testing the units entails becoming sufferers with masks that management the gases they breathe, whereas concurrently taking pulse oximetry readings and samples of arterial blood which might be fed right into a extremely correct measuring machine, invented by the Hypoxia Lab’s late founder, John Severinghaus.
Bickler, who evinces the bemused skepticism of a seasoned automotive mechanic when discussing the scores of units his lab has examined, stated “you can’t always trust what the manufacturers say.”
Their knowledge, he stated, ranges from “completely inaccurate” to “obtained under absolutely ideal conditions, nothing like a real-world performance.”
During the pandemic, a medical charity approached the lab about donating 1000’s of oximeters to poor nations. The oximeters it had chosen “weren’t very good,” he stated. After that, the lab arrange its own ratings page, a form of Consumer Reports for pulse oximeters.
According to its exams, some costly units don’t work; a number of of the $35 devices are more practical than opponents costing $350. Over a 3rd of the marketed units the lab has examined don’t meet present FDA requirements, in response to the positioning.
To examine whether or not real-world exams of oximeters are possible, the FDA funded a UCSF research that has recruited about 200 intensive care unit sufferers. The knowledge from the research is being ready to bear peer evaluate for publication, Bickler stated.
He stated the lab didn’t heat the palms of sufferers within the research, which is the customary follow of producers once they check their units. Warming assures higher circulation within the finger the machine is hooked up to.
“It affects the signal-to-noise ratio,” Bickler stated. “Remember when car radios had AM stations, and you’d get a lot of static? That’s what poor perfusion does — it causes noise, or static that can obscure a clear signal from the device.”
Hypoxia Lab scientists — and medical doctors in the actual world — don’t heat sufferers’ palms. But “the industry people can’t agree on how to handle it,” he stated.
Masimo, an organization that claims it has the most accurate pulse oximeters in the marketplace, would fortunately adjust to any FDA steerage, Daniel Cantillon, Masimo’s chief medical officer, stated in an interview.
How Much To Fix the Problem?
The easiest units, in response to the Hypoxia Lab, cost $6,000 or more. That factors to a different downside.
With higher accuracy, “you are going to reduce patient access to devices for a large proportion of the world that simply can’t afford them,” Lipnick stated.
Even if the FDA can’t please everybody, its anticipated name for extra folks with darker pores and skin in oximetry exams will “assure there’s real diversity in the development and testing of those devices before they come to market,” Lipnick stated. “That bar has been too low for decades.”
It is tough to evaluate hurt to people from defective oximeter readings, as a result of these errors are sometimes one think about a sequence of occasions. But studies at Johns Hopkins University and elsewhere indicated that sufferers whose oxygen depletion wasn’t observed — probably 1000’s of them — had delayed remedy and worse outcomes.
Already, Aboelata stated, a number of producers — Zewa Medical Technology, Veridian Healthcare, and Gurin Products — have responded to the Roots Community Health lawsuit by together with warnings about their units’ limitations.
There’s not a lot she and different clinicians can do in each day follow, she stated, apart from set up a baseline studying with every new affected person and be looking out for notable drops. Hospitals produce other instruments to examine oxygen ranges, however appropriate readings are important for outpatient care, she stated. In 2022, Connecticut enacted a regulation banning insurers from denying dwelling oxygen or different companies based mostly solely on pulse oximetry readings.
But “adapting around the crappy device isn’t the solution,” stated Theodore Iwashyna, the Johns Hopkins Bloomberg School of Public Health professor who co-authored the New England Journal of Medicine article. “A less crappy device is the solution.”
Arthur Allen:
[email protected],
@ArthurAllen202
Related Topics
src=”//platform.twitter.com/widgets.js” charset=”utf-8″>