Marisa Taylor, Kaiser Health News
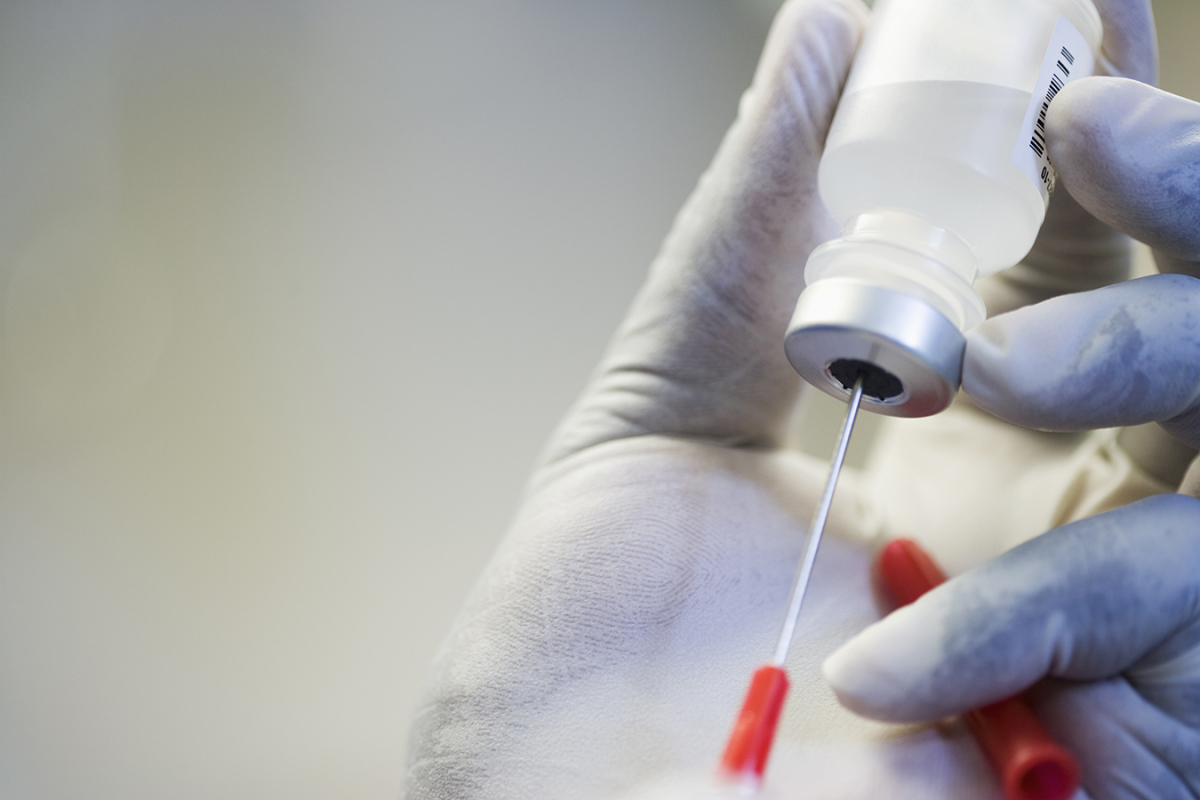
Recent revelations that a U.S. researcher injected Americans along with his experimental herpes vaccine with out routine security oversight raised an uproar amongst scientists and ethicists.
Not solely did Southern Illinois University researcher William Halford vaccinate Americans offshore, he injected different contributors in U.S. hotel rooms with out Food and Drug Administration oversight or perhaps a medical license. Since then, a number of contributors have complained of side effects.
But don’t count on the disclosures after Halford’s dying in June to set off important institutional modifications or authorities response, analysis specialists say.
“A company, university or agency generally does not take responsibility or take action on their own to help participants, even if they’re hurt in the trial,” mentioned Carl Elliott, a professor within the Center for Bioethics on the University of Minnesota. “These types of cases are really a black hole in terms of accountability.”
The federal authorities as soon as scrutinized and even froze analysis at universities after studying of such controversies. Now, specialists mentioned, the oversight businesses are likely to keep away from motion even within the face of probably the most outrageous abuses.
Experts mentioned the U.S. regulatory businesses are particularly unprepared to cope with off-the-grid experiments like Halford’s. He recruited topics via Facebook and in some instances didn’t require signed consent kinds, or knowledgeable contributors outright that the experiments flouted FDA oversight. These sufferers, many who battle with persistent, painful herpes, proceeded anyway of their quest for a treatment. After Halford’s offshore trial, Peter Thiel, a libertarian and adviser to President Donald Trump, pitched in tens of millions of dollars for future analysis.
“This is experimentation in the 21st century: heavily embedded in social media and combined with a hostility to regulatory oversight,” mentioned Arthur Caplan, director of the Division of Medical Ethics at New York University’s School of Medicine. “How is the government going to manage subjects, researchers and investors who don’t like regulations?”
SIU officers initially disregarded any questions on Halford’s strategies, saying the college didn’t have accountability for the offshore vaccinations of the 20 contributors within the trial as a result of he fashioned an unbiased firm to conduct the trial in St. Kitts and Nevis.
SIU finally launched an inquiry final August, greater than a month after Kaiser Health News started asking questions on Halford’s strategies. The investigation is ongoing, though a preliminary inquiry discovered Halford’s strategies to be in “serious noncompliance” with college guidelines and U.S. rules.
Experts say the college ought to contact all contributors who have been injected with the vaccine and provide assist to those that are affected by unintended effects. But they level out that the university has potential conflicts of interest when scrutinizing its function in Halford’s analysis. SIU shared within the vaccine patent with Halford’s firm, and medical college administration officers touted his work when Rational Vaccines benefited from Thiel’s funding.
In ongoing reporting, KHN realized that Halford injected a gaggle of individuals in two lodge rooms close to his lab in Springfield, Ill., in 2013, three years earlier than he started experiments offshore. According to emails and one of many contributors, many electronic mail exchanges with contributors have been despatched from Halford’s college electronic mail account. He used the college cellphone and referred to a graduate pupil as helping within the experiment and utilizing the lab.
One participant has advised the FDA he believes he suffers from antagonistic results from the vaccine.
Yet Rational Vaccines continues to claim the vaccine is protected and efficient, regardless that Halford’s information from his human topic experiments haven’t been revealed in a good scientific journal. Since the controversy, the corporate has taken down its web site.
“This researcher basically violated all of the regulatory requirements and ethical principles guiding human subject research,” mentioned Michael Carome, a health care provider who directs the well being analysis group for the nonprofit advocacy group Public Citizen. “Unfortunately, it seems everyone is trying to distance themselves here to avoid legal liability and a public relations embarrassment.”
The FDA, which has declined to touch upon this case, might have jurisdiction, however it hardly ever takes aggressive motion on behalf of human topics, specialists mentioned. The FDA has had restricted contact with two contributors who’ve filed complaints alleging unintended effects from the vaccine.
“The FDA is just not set up to handle this,” NYU’s Caplan mentioned.
The federal Office for Human Research Protections (OHRP), which displays how human topics are handled in trials, might select to conduct an unbiased investigation. The company would have jurisdiction as a result of the college had pledged to comply with human topic security protocols for all analysis, even when it was funded privately.
Experts, nevertheless, stay skeptical that OHRP, an company within the Department of Health and Human Services, would assume a distinguished function in investigating the case based mostly on the company’s monitor file.
OHRP requested the college for an evidence after KHN first reported that Halford didn’t ask for routine security oversight from an institutional evaluate board.
OHRP as soon as took on human topic violations that occurred throughout non-federally funded analysis and in instances the place the FDA asserted jurisdiction, Carome mentioned.
Now, the company usually chooses to remain out of non-federally funded trials and defers to the FDA, he mentioned.
As a consequence, its public response to allegations of analysis abuse has plummeted, specialists mentioned.
The company’s public assessments of analysis misconduct peaked in 2002, when it issued greater than 100 “determination letters.” That quantity has steadily declined. This yr, it has issued one.
“A single letter in one year is extraordinary,” mentioned Carome, who was the company’s affiliate director for regulatory affairs from 2002 to 2010. “OHRP’s compliance oversight activities are moribund.”
“The end result is the federal watchdog for human subject protections is ineffective in its role in investigating complaints” and stopping violations, he mentioned.
OHRP maintains it has been utilizing different “more efficient” approaches. Rather than robotically opening a case and issuing a willpower letter, the company is “working more closely with complainants and institutions to address some of the concerns raised about human subject research,” mentioned an HHS spokesperson, who declined to be named citing company coverage.
“But … in situations where something seriously wrong occurred, or subjects were harmed, OHRP does take action,” the spokesperson mentioned.
OHRP hasn’t taken high-profile, aggressive motion in years, mentioned specialists who pointed to the federal government’s suspension of federally funded analysis at Duke University and Johns Hopkins University greater than a decade in the past.
The federal authorities’s unresponsiveness in instances of privately funded analysis turned extra pronounced beneath the Obama administration, specialists mentioned.
In the waning days of the Obama administration, the federal authorities accepted the primary main overhaul of rules surrounding human trials in 40 years. The ensuing modifications to federally funded trials included making consent kinds extra concise and clear.
Laura Stark, affiliate professor at Vanderbilt University’s Center for Medicine, Health and Society, mentioned that in federally funded trials the measure “shifts the balance towards protecting research participants rather than protect[ing] institutions against liability.”
However, the rule doesn’t deal with privately funded trials.
“If [the] organization and studies are privately funded, then they are not beholden to the law,” Stark mentioned
As extra universities started dropping their pledges to comply with security protocols for all analysis, it had already change into troublesome for the company to claim jurisdiction. Meanwhile, OHRP’s compliance employees dwindled from six workers in 2008 to 2 in 2017. The lack of accountability for privately funded analysis is unlikely to vary, specialists say.
Stark referred to as Halford’s analysis “a potential harbinger of the future of medical research given the increase in private funding and the unlikely prospect of updating the regulations again anytime soon.”
Compounding the issue, the United States is the one developed nation that doesn’t assure medical take care of these injured in medical trials, specialists mentioned.
When contributors declare damage, they usually are advised to file claims with their insurance coverage corporations, an unimaginable endeavor, mentioned NYU’s Caplan.
“We still haven’t figured out how to compensate people who say they are injured in research,” he mentioned. “In cases like these, in which subjects are claiming harm from a cuckoo experiment, the system is set up to punish the institution rather than give redress to the subject. Their only route then is to sue.”