Use This Story This story will be republished at no cost (details).
The Food and Drug Administration introduced a sweeping plan on Friday to assessment and handle the protection of surgical staplers, together with a brand new examination of seven years’ value of hidden reviews highlighted Thursday in a Kaiser Health News investigation.
In a letter despatched to health care providers Friday, the FDA stated it would convene an advisory assembly on the protection of the units and signaled that it would reclassify surgical staplers to place them underneath tighter management. The company additionally stated it plans to problem proposed suggestions to firms that make the units, that are utilized in numerous surgical procedures.
The FDA additionally acknowledged in its letter that “we are aware that many more device malfunction reports during this time frame” have been submitted as “summary reports,” which go to the FDA however aren’t included within the public database often called MAUDE (the Manufacturer and User Facility Device Experience). The company stated it “is conducting an analysis” of these abstract reviews and the outcomes might be made obtainable to the general public.
The KHN investigation discovered that in 2016 alone almost 10,000 surgical stapler malfunction reviews flowed into the interior FDA database that few patient-safety consultants who have been contacted knew existed. The public database that yr disclosed fewer than 100 accidents or malfunctions.
The secretive reviews on staplers have been a part of a much wider FDA program, collectively known as “alternative summary reporting.” Since the beginning of 2016, greater than 1.1 million severe harm or malfunction reviews went into the interior database as a substitute of being disclosed intimately in MAUDE, which is extensively utilized by docs and researchers to trace issues.
The FDA has stated “about 100” units have had particular exemptions to report incidents that method however confirmed the id of only some units to KHN. The company stated it revoked many of the reporting exemptions in 2017 as a brand new summary-reporting program took form and requested system makers to file reviews noting publicly that abstract information was being submitted along with information that will be stored contained in the company.
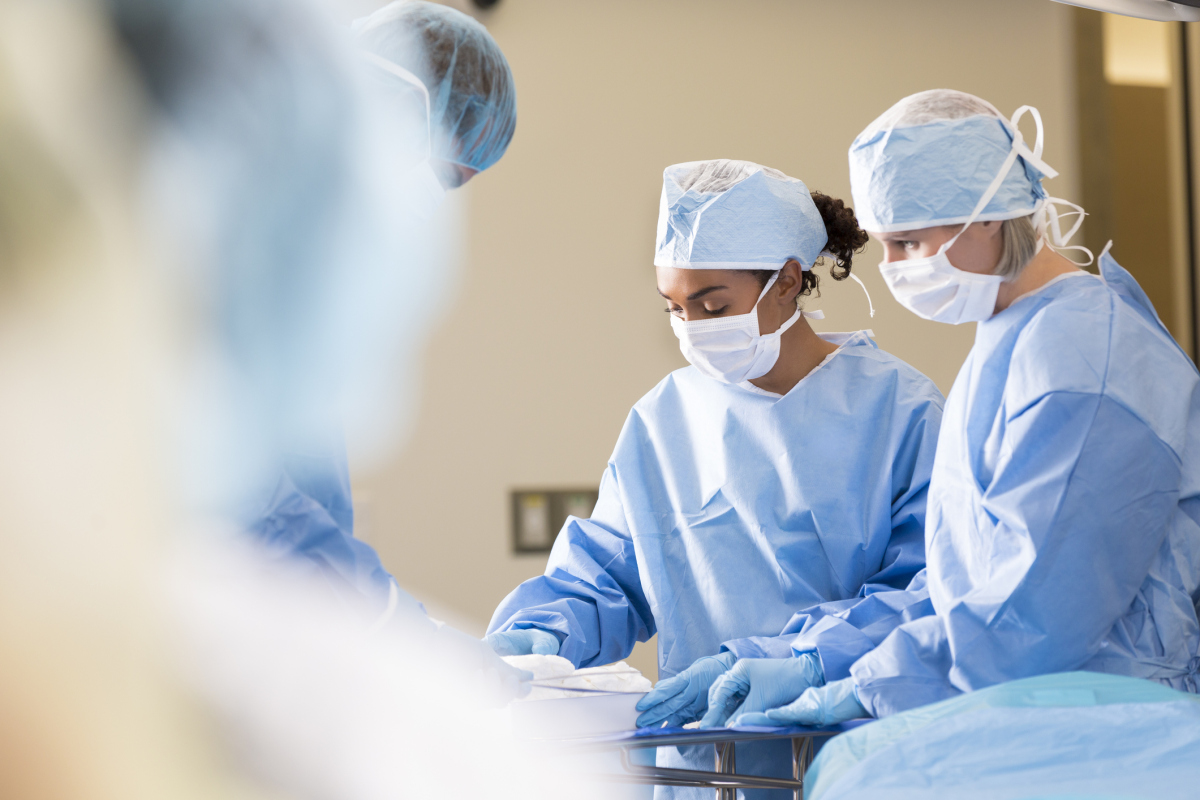
After Dr. Douglas Kwazneski witnessed a surgical stapler malfunction, he surveyed main surgeons and found that greater than two-thirds had skilled a stapler malfunction, or knew a peer who did.(Kendra Stanley-Mills for KHN)
The information report highlighted the work of Dr. Douglas Kwazneski, who skilled a stapler malfunction then turned to FDA information and noticed “there was nothing” — a shock that on the time “seemed like a cover-up,” Kwazneski stated.
Surgical staplers reduce and seal blood vessels and tissue, typically throughout minimally invasive surgical procedures. When the staplers fail to fireplace, sufferers have bled profusely and suffered dire accidents or loss of life. The FDA stated stapler malfunctions or misuse can result in “bleeding, sepsis, tearing of internal tissues and organs, increased risk of cancer recurrence, and death.”
The FDA has granted reporting exemptions through the years for each surgical staplers and staples, company information present.
Stapler maker Medtronic confirmed that its reporting exemption for surgical staples resulted in mid-2017. FDA information present that its public reviews of accidents and malfunctions soared, from about 1,000 in 2015 to 11,000 final yr. Ethicon, which additionally makes staplers, stated it has not used the reporting exemption.
Email Sign-Up
Subscribe to KHN’s free Morning Briefing.
In the company’s Friday announcement, Dr. William Maisel, chief medical officer within the FDA’s Center for Devices and Radiological Health, stated the company’s assessment of stapler incidents is ongoing. “But we know these devices provide important benefits for patients undergoing surgery, so it’s important for us to continue to educate providers about the devices’ safety and risk.”
“Improving the safety of surgical staplers and implantable staples is a top priority for the FDA, and we believe our forthcoming draft guidance to industry and planned advisory committee meeting will advance those efforts,” Maisel stated.
The company’s announcement stated that public reviews of hurt by staples and staplers present “that from January 1, 2011 to March 31, 2018, the FDA received more than 41,000 individual medical device reports for surgical staplers and staples for internal use, including 366 deaths, more than 9,000 serious injuries, and more than 32,000 malfunctions.”
The company additionally stated that altering the classification of surgical staplers might enable the company to require “performance testing of various mechanical features” and particular labeling.
Use This Story This story will be republished at no cost (details).
Christina Jewett: [email protected]”>[email protected], @by_cjewett
Related Topics Health Industry FDA Medical Devices src=”http://platform.twitter.com/widgets.js” charset=”utf-Eight”>