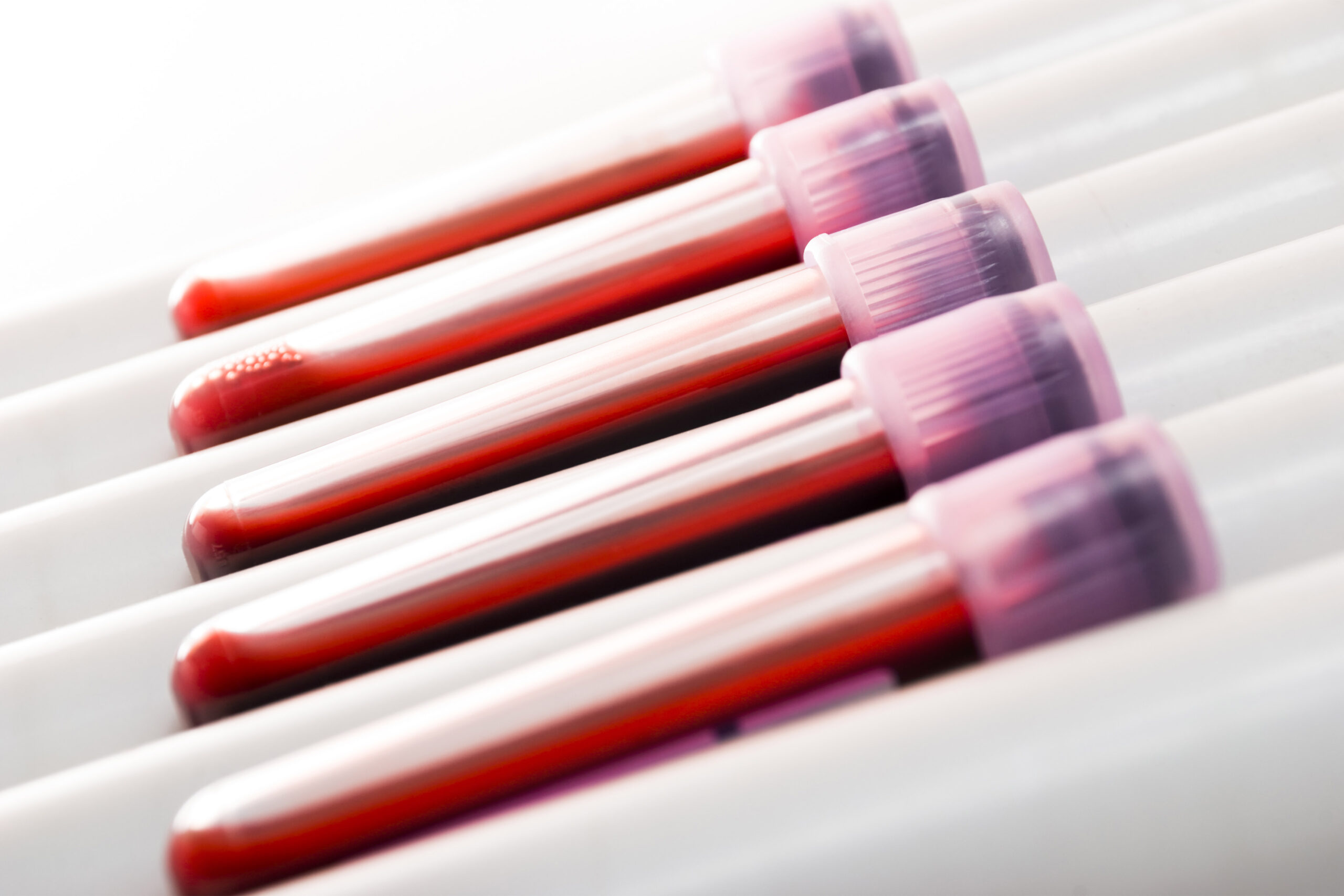
An organization that makes assessments for lead poisoning has agreed to resolve legal expenses that it hid for years a malfunction that resulted in inaccurately low outcomes.
It’s the most recent in a long-running saga involving Massachusetts-based Magellan Diagnostics, which is able to pay $42 million in penalties, based on the Department of Justice.
While lots of the fault-prone gadgets had been used from 2013 to 2017, some had been being recalled as late as 2021. The Justice Department stated the malfunction produced inaccurate outcomes for “potentially tens of thousands” of kids and different sufferers.
Doctors don’t take into account any degree of lead within the blood to be protected, particularly for youngsters. Several U.S. cities, together with Washington, D.C., and Flint, Michigan, have struggled with widespread lead contamination of their water provides within the final 20 years, making correct assessments important for public well being.
It’s potential defective Magellan kits had been used to check kids for lead publicity into the early 2020s, based mostly on the recall in 2021. Here’s what mother and father ought to know.
What assessments had been affected?
The inaccurate outcomes got here from three Magellan gadgets: LeadCare Ultra, LeadCare II, and LeadCare Plus. One, the LeadCare II, makes use of finger-stick samples primarily and accounted for greater than half of all blood lead assessments carried out within the U.S. from 2013 to 2017, based on the Justice Department. It was usually utilized in doctor workplaces to verify kids’s lead ranges.
The different two is also used with blood drawn from a vein and will have been extra widespread in labs than physician’s workplaces. The firm “first learned that a malfunction in its LeadCare Ultra device could cause inaccurate lead test results – specifically, lead test results that were falsely low” in June 2013 whereas in search of regulatory clearance to promote the product, the DOJ stated. But it didn’t disclose that data and went on to market the assessments, based on the settlement.
The company stated 2013 testing indicated the identical flaw affected the LeadCare II system. A 2021 recall included most of all three forms of take a look at kits distributed since October 27, 2020.
The firm stated in a press release saying the decision that “the underlying issues that affected the results of some of Magellan’s products from 2013 to 2018 have been fully and effectively remediated,” and that the assessments it at the moment sells are protected.
What does a falsely low outcome imply?
Children are sometimes examined throughout pediatrician visits at age 1 and once more at age 2. Elevated lead ranges can put youngsters susceptible to developmental delay, decrease IQ, and different issues. And signs, reminiscent of stomachache, poor urge for food, or irritability, might not seem till excessive ranges are reached.
Falsely low take a look at outcomes may imply mother and father and physicians had been unaware of the issue.
That’s a priority as a result of remedy for lead poisoning is, initially, primarily preventive. Results exhibiting elevated ranges ought to immediate mother and father and well being officers to find out the sources of lead and take steps to forestall continued lead consumption, stated Janine Kerr, well being educator with the Virginia Department of Health’s Childhood Lead Poisoning Prevention Program.
Children will be uncovered to guide in a wide range of methods, together with by consuming water contaminated with lead from previous pipes, reminiscent of in Flint and Washington; ingesting lead-based paint flakes usually present in older properties; or, as reported lately, consuming some brands of cinnamon-flavored applesauce.
What ought to mother and father do now?
“Parents can contact their child’s pediatrician to determine if their child had a blood lead test with a LeadCare device” and focus on whether or not a repeat blood lead take a look at is required, stated Maida Galvez, a pediatrician and professor on the Icahn School of Medicine at Mount Sinai in New York.
During an earlier recall of some Magellan gadgets, in 2017, the Centers for Disease Control and Prevention recommended that patients be retested in the event that they had been pregnant, nursing, or kids youthful than 6 and had a blood lead degree of lower than 10 micrograms per deciliter as decided by a Magellan system from a venous blood draw.
The 2021 recall of Magellan gadgets advisable retesting kids whose outcomes had been lower than the present CDC reference degree of three.5 micrograms per deciliter. Many of these assessments had been of the finger-stick selection.
Kerr, on the Virginia well being division, stated her company has not had many calls about that recall.
The finger-stick assessments “are not that widely used in Virginia,” stated Kerr, including that “we did get a lot of questions about the applesauce recall.”
In any case, she stated, the “best course of action for parents is to talk with a health care provider.”
Julie Appleby:
[email protected],
@Julie_appleby
Related Topics
src=”//platform.twitter.com/widgets.js” charset=”utf-8″>